Characterization of the peripheral immune compartment during fasting in healthy volunteers and MS patients
Scientific interest within the context of the graduate college:
Diet is an important factor for a healthy life. For the most part of human history, the next meal was not a given. Hence, there was a strong selection pressure for adaptation to periods of no or low food consumption during our evolution. Consequently, today’s excessive calorie intake, as it is typical for diets in the western world, results in increasing occurrence of systemic inflammation and widespread diseases. In contrast, calorie restriction has been shown to improve numerous chronic diseases and to prolong the healthy lifespan. In my group, we are re-thinking health in the context of evolutionary adaptation to low food energy intake. Specifically, we focus on the identification of cellular and molecular mechanisms how reduced calorie intake maintains health, prevents and improves inflammatory diseases, and prolongs healthy life.
Project description:
The focus of my group is to understand the cellular and molecular mechanisms how reduced calorie intake regulates homeostasis and function of the immune system. Recently, we have found that fasting drastically reduces the number of circulating pro-inflammatory monocytes in the blood of humans and mice.1 Interestingly, monocytes accumulated in the bone marrow. Our preliminary data suggest that this is due to the inhibition of monocyte egress from the bone marrow as well as to recruitment from the blood circulation. This phenomenon can be observed not only for monocytes but also for specific other immune cell populations such as naïve B cells and memory T cells.2,3 Intriguingly, memory T cells that have been re-located to the bone marrow display functional modulation and improvement upon egress and re-circulation.2 Hence, our research question is: Are monocytes functionally modified in the bone marrow during fasting? To answer this question, we will establish a monocyte adoptive transfer model in mice and apply next generation sequencing to monocytes in the periphery and the bone marrow. Thus, this project offers the opportunity to the student to develop bench working skills as well as getting familiar with the computational analysis of large transcriptomic datasets.
The aim of the study is to analyze the transcriptional profile of monocytes that returned to the bone marrow from the periphery during fasting.
WP1: Establishing an adoptive monocyte transfer model into fed and fasted mice. It is crucial to the study that monocytes returning to the bone marrow during fasting can be clearly identified. Therefore, we will isolate Ly-6C+ monocytes from the spleen of CD45.1+ mice using the Miltenyi monocyte isolation kit (Fig. 1). Isolated monocytes will be transferred into CD45.2+ mice that will be fed or fasted for 16 hours via intravenous injection. After 4 hours, bone marrow, blood, spleen, and additional organs will be harvested and analyzed for transferred CD45.1+ monocytes using multicolor flow cytometry. Hence, CD45.1/CD45.2 discrimination will enable us to identify and quantify monocytes that are recruited from the blood to the bone marrow. We already obtained the approval of the responsible state office for the animal experiments.
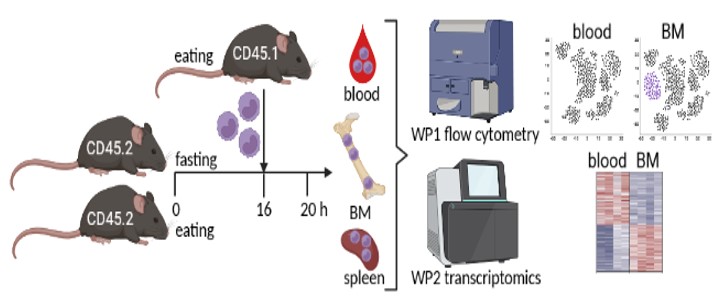
BM = bone marrow.
WP2: Monocyte transcriptomics and computational analysis. In WP2 we will transfer splenic CD45.1+ monocytes into fed and fasted mice as described in WP1 for transcriptional analysis. We will analyze CD45.1+ monocytes that were recruited to the bone marrow, as well as CD45.1+ monocytes that remained in the blood circulation or homed to the spleen. The comparison between monocytes that returned to the bone marrow in fed vs. fasted mice will identify specific fasting-induced modifications. We will also include a monocyte sample from the spleen of a fed mouse for analysis of the pre-transfer state. We will enrich CD45.1+ monocytes from the respective organs using the Miltenyi monocyte isolation kit and subsequently use flow cytometry to sort to high purity for sequencing. Because we do not expect to yield high cell numbers, we will use ultra-low input sequencing approaches and multiplex samples to save resources. The computational analysis will include among others: differentially expressed genes between bone marrow and peripheral monocytes during fasting, gene ontology, KEGG and pathway analysis, upstream regulators of transcriptional changes, prediction of modified cellular functions, analysis of cellular metabolic and transcriptional networks as well as intercellular communication using Ingenuity Pathway analysis and R tools as we have done before.1
In summary, we will investigate functional modifications of monocytes in the bone marrow during fasting using a monocyte transfer model in combination with transcriptional analysis. The results could indicate how fasting reduces the pro-inflammatory potential of monocytes and, thereby, prevents inflammatory disease and prolongs healthy life.
References
- Jordan S, Tung N, Casanova-Acebes M, […], Berres ML, Gallagher EJ, Merad M. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 2019; 178:1102-1114 e1117. doi: 10.1016/j.cell.2019.07.050.
- Collins, N, Han SJ, Enamorado M, […], McGavern DB, Schwartzberg PL, Belkaid Y. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell. 2019; 178:1088-1101 e1015. doi: 10.1016/j.cell.2019.07.049.
- Nagai M, Noguchi R, Takahashi D, […] Takubo K, Dohi T, Hase K. Fasting-Refeeding Impacts Immune Cell Dynamics and Mucosal Immune Responses. Cell. 2019; 178:1072-1087 e1014. doi: 10.1016/j.cell.2019.07.047.
The role of the epithelial transcription factor NF-κB in homeostasis, development and regeneration of chronic inflammatory bowel diseases
Scientific interest within the context of the graduate college:
Our group “Signal Transduction in Health and Disease” (Department of Gastroenterology and Hepatology, Charité Virchow) aims to understand the molecular mechanisms involved in tissue homeostasis, inflammation, and resolution of inflammation. Our main focus is on the transcription factor NF-κB and its role in the intestinal epithelium. Recent studies showed that crosstalk between epithelium and immune cells changes in health and disease, and that is in part due to altered functions of NF-κB. Our group aims to decipher that crosstalk and identify the changes. Our research is highly interdisciplinary and spans different fields including biochemistry, immunology, stem cell biology, and cancer biology.
Project description:
NF-κB is deemed the master regulator of pro-inflammatory response and its activation directly correlates with severity of inflammation in Inflammatory Bowel Disease (IBD).1 Although in immune cells, NF-κB plays largely pro-inflammatory role, in intestinal epithelium its function is poorly understood. Many IBD therapies rely on suppression of NF-κB. These therapies however inhibit NF-κB across different tissues and cell types, including healthy ones. This can lead to many adverse effects, including susceptibility to infection and carcinogenesis. We have recently shown that a single stress stimulus sequentially activates functionally distinct transcriptomes of NF-κB: anti-apoptotic and pro-inflammatory.2-3 We also showed that in homeostasis, NF-κB directs differentiation of stem cells.4 To identify therapies that specifically target only the pro-inflammatory NF-κB, it is critical to decipher what roles NF-κB plays in health, in acute inflammation, and finally in mucosal healing.
Based on our recently published and unpublished data we propose that acute, immediate activation of NF-κB in intestinal epithelium is necessary for resolution and for regaining homeostasis. To address this hypothesis we will use single-cell datasets, intestinal organoids, and transgenic mouse models. We have recently established transgenic mice that provide a specific readout of NF-kB activity as well as mice with either specific suppression or constitutive activation of NF-kB in intestinal epithelium.2-4 These tools could enable us, for the first time, to determine where and how NF-κB can be targeted to both prevent chronic inflammation or to promote resolution.
Our key aims are:
1) To identify which cells activate NF-kB under homeostasis versus in inflammation, and to determine which transcriptional programs NF-kB initiates in these cells.
2) To determine how repression or constitutive activation of NF-kB affects intestinal homeostasis, acute inflammation, and resolution.
3) Identify which singling pathways can be targeted to safeguard homeostasis or promote resolution of inflammation.
References
- Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008; 263:591-596. doi: 10.1111/j.1365-2796.2008.01953.x.
- Kolesnichenko M, Mikuda N, Höpken UE, […], Schmidt-Ullrich R, Schmitt CA, Scheidereit C. Transcriptional repression of NFKBIA triggers constitutive IKK- and proteasome-independent p65/RelA activation in senescence. EMBO J. 2021; 40:e104296. doi: 10.15252/embj.2019104296.
- Mikuda N, Schmidt-Ullrich R, Kärgel E, […], Scheidereit C, Kühl AA, Kolesnichenko M. Deficiency in IκBα in the intestinal epithelium leads to spontaneous inflammation and mediates apoptosis in the gut. J Pathol. 2020; 251:160-174. doi: 10.1002/path.5437.
- Brischetto C, Krieger K, Klotz C, […], Heuberger J, Scheidereit C, Schmidt-Ullrich R. NF-κB determines Paneth versus goblet cell fate decision in the small intestine. Development. 2021; 148:dev199683. doi: 10.1242/dev.199683.
Cellular mechanisms underlying the pathogenetic role of the primary cilium in pulmonary arterial hypertension
Scientific interest within the context of the graduate college:
Maintenance or restoration of vascular homeostasis are critical determinants of health throughout life. Of late, the primary cilium has been identified as a key regulator of vascular homeostasis and regeneration. While impairment of primary ciliary structure and signaling can promote vascular disease and vascular remodeling, strategies aiming to preserve or restore ciliary function emerge as novel therapeutic approaches to maintain vascular health.
Project description:
Physiologically, homeostatic signaling in response to biochemical and biomechanical cues within the pulmonary vascular wall warrants the integrity of the vascular structure of the lung and allows for its adaptation to changing requirements throughout life. Dysregulated signaling in or between pulmonary artery endothelial and smooth muscle cells, on the other hand, causes progressive adverse remodeling of the pulmonary vasculature, resulting in increased pulmonary vascular resistance, pulmonary hypertension, and ultimately death due to right heart failure. Understanding the cellular signaling mechanisms that maintain or restore vascular homeostasis in the lung is therefore critical for the prevention or therapy of pulmonary hypertension, and for the preservation of an intact vasculature throughout life up to an old age.
Ongoing research in our group has identified the primary cilium as a novel key regulator of vascular homeostasis in the lung. Unlike the motile cilia found e.g. on the respiratory epithelium or in the Fallopian tube, the primary cilium is a singular organelle (one per each cell) that is found on almost every cell type in the body. The primary cilium extends as a protrusion of the cell membrane into the extracellular space where it functions as a mechano- and chemosensor, while its base acts as a hub for numerous cellular signaling pathways. We have shown that the primary cilium maintains the cells of the pulmonary vasculature in a physiological quiescent state. Loss of the primary cilium, however, causes pathological proliferation and migration of both cell types. Importantly, we have identified such loss of the primary cilium as a hallmark of pulmonary blood vessels in patients with pulmonary hypertension. These findings fuel the intriguing hypothesis that strategies aiming to preserve or restore primary cilium structure and function may present a novel therapeutic approach to maintain lung vascular health and to reverse maladaptive vascular remodeling. In order to test this hypothesis, the present project will address the following questions:
Question 1: Which mechanisms regulate the integrity of the primary cilium in lung vascular endothelial and smooth muscle cells? Based on previous work by us and others, we will focus here specifically on signaling axes via mammalian target of rapamycin (mTOR) and aurora kinase A. Specifically, we will test whether pharmacological or genetic modulation of these pathways can maintain primary ciliary integrity in preclinical models of pulmonary hypertension.
Question 2: Can strategies aimed to restore ciliary integrity preserve lung vascular homeostasis? We have recently patented a novel intervention for rescue of the primary cilium in pulmonary hypertension. We will test whether this strategy, as well as interventions identified in Aim 1 can restore vascular homeostasis in preclinical models of pulmonary hypertension both in vitro and in vivo.
References
- Fan Y, Gu X, Zhang J, […], Solymosi P, Kwapiszewska G, Kuebler WM. TWIST1 drives smooth muscle cell proliferation in pulmonary hypertension via loss of GATA-6 and BMPR2. Am J Respir Crit Care Med. 2020; 202: 1283-1296. doi: 10.1164/rccm.201909-1884OC.
- Dummer A, Rol N, Szulcek R, […], DeRuiter MC, Goumans MJ, Hierck BP. Endothelial dysfunction in pulmonary arterial hypertension: loss of cilia length regulation upon cytokine stimulation. Pulm Circ. 2018; 8: 2045894018764629.doi: 10.1177/2045894018764629.
The role of Muc5b in lung homeostasis and onset and progression of interstitial lung disease
Scientific interest within the context of the graduate college:
Mucociliary clearance is the primary innate defense mechanism of the lung and crucial to maintain lung homeostasis and health. Mucociliary clearance relies on motile cilia on the surface of epithelial cells and a protective mucus gel layer entrapping particles and pathogens to be cleared from the lungs. Recent evidence suggests that the secreted mucin MUC5B that is crucial for the formation of the mucus gel and proper mucociliary clearance is also implicated in the pathogenesis of interstitial lung disease (ILD).1 ILD can affect children and adults and is characterized by interstitial inflammation, rapid progression of pulmonary fibrosis and subsequent disruption of the alveolar gas exchange ultimately leading to respiratory failure. The understanding of the pathogenesis remains limited and ILD is usually diagnosed in advanced stages when irreversible lung damage has already occurred. Further, only limited therapeutic options are available, which so far, cannot prevent progression of pulmonary fibrosis. By conditional deletion of Nedd4-2 (Nedd4-2-/-) in lung epithelial cells of mice, we recently generated the first mouse model that develops spontaneous pulmonary fibrosis sharing key features with ILD patients allowing us to study lung homeostasis at baseline and early dysregulation leading to the development of interstitial lung disease.2,3
Project description:
The aim of this translational research project is to investigate the role of Muc5b in the onset and progression of interstitial lung disease in conditional Nedd4-2-/- mice. For this purpose, double knockout mice deficient for Nedd4‑2 and Muc5b will be generated and their lung phenotype comprehensively studied at different stages of disease progression to identify early changes in lung homeostasis and pathways that promote disease progression and may serve as therapeutic targets. Clinical parameters such as inflammation markers, histology, lung function, as well as mucus properties will be investigated in order to gain insight into the relationship between altered mucus properties and disease pathogenesis. Further, our findings will be validated in lung biopsies and serum samples from patients with ILD. In addition to basic molecular biology laboratory work, this experimental MD thesis also applies state-of-the-art mouse lung function, mucus rheological measurements, and high content microscopy.
References
- Evans CM, Fingerlin TE, Schwarz MI, […], Warg L, Yang IV, Schwartz DA. Idiopathic Pulmonary Fibrosis: A Genetic Disease That Involves Mucociliary Dysfunction of the Peripheral Airways. Physiol Rev. 2016; 96:1567-1591. doi: 10.1152/physrev.00004.2016.
- Duerr J, Leitz DHW, Szczygiel M, […], Beers MF, Klingmüller U, Mall MA. Conditional deletion of Nedd4-2 in lung epithelial cells causes progressive pulmonary fibrosis in adult mice. Nat Commun. 2020; 11:2012. doi: 10.1038/s41467-020-15743-6.
- Leitz DHW., Duerr J, Mulugeta S, […], Dalpke AH, Beers MF, Mall MA. Congenital Deletion of Nedd4-2 in Lung Epithelial Cells Causes Progressive Alveolitis and Pulmonary Fibrosis in Neonatal Mice. Int J Mol Sci. 2021; 22:6146. doi: 10.3390/ijms22116146.
How does NK cell memory develop in humans?
Scientific interest within the context of the graduate college:
The innate immune system matures early on along with tissue development. In particular, innate lymphoid cells (ILCs, including Natural Killer (NK) cells, already colonize tissues during fetal life and acquire their effector functions already pre-natally. However, exposure to different environmental signals, including microbial infection, can still remodel ILCs and leave permanent epigenetic marks. Our group focuses on delineating transcriptionally and epigenetically the immune functional programs pre-wired during development as well as those emerged and imprinted after exposure to environmental stimuli, in particular persistent virus, thereby setting the threshold of our immune fitness and tolerance to tissue damage. Our aim is to understand the key homeostatic checkpoints, which, once altered, can initiate inflammatory circuits and lead to disease.
Project description:
Aim 1: Analysis of ex vivo dynamics of NK cell clonality and epigenetic remodeling in response to HCMV. In this part of the project, we aim to understand how NK cell clonal expansions are generated and maintained. To this aim, we will perform the following experiments:
A) Lineage tracing over time of human NKG2C+ and NKG2C– NK cells derived from peripheral blood of HCMV+ and HCMV– healthy individuals by performing scATACseq/scRNAseq and analysis of mitochondrial mutations (DOGMAseq).5
B) Lineage tracing over time of human NKG2C+ and NKG2C– donor NK cells in patients undergoing hematopoietic stem cell transplantation and HCMV reactivation (established cooperation with Hematology Charité, Prof L. Büllinger). NK cells will be isolated from peripheral blood of patients between day 10 and day 30 after transplantation, during HCMV reactivation (typically around day 30), and at later time points (day 60-120). DOGMAseq will be performed as in 1A. Moreover, clinical and laboratory parameters along with sequencing of HCMV-relevant proteins will be assessed.
Aim 2: Analysis of in vitro dynamics of NK cell clonality and epigenetic remodeling in response to HCMV-derived signals. In this part of the project, we aim to understand the signals driving NK cell clonal expansion. To this aim, we will mimic NK cell clonal expansion in vitro by culturing human NKG2C+ “naive” NK cells derived from peripheral blood of HCMV– healthy individuals in the presence of signals mimicking HCMV stimulation (NKG2C peptide ligands, CD2 costimulation, and pro-inflammatory cytokines). Proliferating NK cells will be sorted at different time points and DOGMA-seq will be performed.
References
- Luetke-Eversloh M, Hammer Q, Durek P, […], Chang HD, Dong J, Romagnani C. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 2014; 10:e1004441. doi: 10.1371/journal.ppat.1004441.
- Hammer Q, Rückert T, Romagnani C. Natural killer cell specificity for viral infections. Nat Immunol. 2018; 19:800-808. doi: 10.1038/s41590-018-0163-6.
- Hammer Q, Rückert T, Borst EM, […], Mashreghi MF, Messerle M, Romagnani C. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol. 2018; 19:453-463. doi: 10.1038/s41590-018-0082-6.
- Ludwig LS, Lareau CA, Ulirsch JC, […], Buenrostro JD, Regev A, Sankaran VG. Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell. 2019; 176:1325-1339.e22. doi: 10.1016/j.cell.2019.01.022.
- Mimitou EP, Lareau CA, Chen KY, Sankaran VG, Regev A, Smibert P. Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells. Nat Biotechnol. 2021; 39:1246-1258. doi: 10.1038/s41587-021-00927-2.
Investigating the impact of nano- and microplastics on hypertensive disease
Scientific interest within the context of the graduate college:
Environmental factors such as nutrition but also pollution can significantly influence the health-to-disease transition. The contact of the environment with the human organism, especially at interfaces such as the intestine and the immune system, is of crucial importance. Often, several harmful environmental influences come together to trigger a disease or promote its development. Through our project, we aim to create a better understanding of environmental factors that promote the development of cardiovascular diseases via immunological mechanisms.
Project description:
Plastic pollution is a major challenge in our today’s society. Micro- and nanoplastic particles have been found in various foods1 and recently the uptake of the particles into our organism has been proven by the discovery of plastic particles in human blood.2 Microplastics have been shown to alter the microbiome and induce inflammation.3-5 Current research thereby focuses mainly on the impact of plastic in healthy organisms. However, a large amount of our population suffers from diseases such as hypertension. In this project, we aim to investigate the impact of Micro- and nanoplastics in the setting of hypertension. The microbiome-immune axis has been shown to be of high relevance in hypertensive-related organ damage.6,7 We aim to explore the impact of plastic particles on all parts of this axis – the microbiome, the immune system, and organ damage by using a murine hypertension model. The project will help to enlighten the risks of plastic pollution for human health.
References
- Paul MB, Stock V, Cara-Carmona J, […], Braeuning A, Sieg, H, Böhmert, L. Micro- and nanoplastics – current state of knowledge with the focus on oral uptake and toxicity. Nanoscale Adv. 2020; 2:4350-4367. doi:10.1039/D0NA00539H.
- Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic pollution in human blood. Environ Int. 2022; 107199. doi: 10.1016/j.envint.2022.107199.
- Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice.Sci Total Environ. 2018; 631-632:449-458. doi: 10.1016/j.scitotenv.2018.03.051.
- Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci Total Environ. 2019; 649:308-317. doi: 10.1016/j.scitotenv.2018.08.353.
- Qiao J, Chen R, Wang M, […], Liu Y, Wu C, Chen C. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale. 2021; 13:8806-8816. doi: 10.1039/d1nr00038a.
- Wilck N, Matus MG, Kearney SM, […], Linker RA, Alm EJ, Muller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017; 551:585-589. doi: 10.1038/nature24628.
- Bartolomaeus H, Balogh A, Yakoub M, […], Muller DN, Stegbauer J, Wilck N. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019; 139:1407-1421. doi: 10.1161/CIRCULATIONAHA.118.036652.
Mechanosensing in macrophages in health and disease
Scientific interest within the context of the graduate college:
Our lab is interested in analyzing immune cells in the tissue context, using state-of-the-art, functional intravital microscopy and histocytometry approaches.1,2,3 In addition to reacting to stimuli from hematopoietic and non-hematopoietic cells, for example via direct cell-cell contacts or via soluble mediators, immune cells are exposed to a variety of other stimuli present in the tissue, among them mechanical cues. The significance of those stimuli for immune cells has not been explored in detail. Recently, a role for the mechanosensor Piezo1 in shaping myeloid cell activation has been identified.4 Here, we aim to understand how mechanical cues in barrier tissues affect the function of myeloid cells. We will focus the lamina propria of the intestine, a tissue that in the healthy organism is constantly subjected to contractions (peristalsis). We hypothesize that this mechanical stimulation impacts on the phenotype of macrophages in the lamina propria. As alterations in peristalsis appear in certain situations, such as parasitic infections, we aim to test to what extent mechanosensing in macrophages affects their function under those conditions.
Project description:
To monitor mechanosensing in vivo, we aim to use a reporter system, which allows for the detection of Ca2+ flux, induced by the mechanosensor Piezo1, in intestinal macrophages by intravital microscopy.5 The objectives for this project are:
Aim 1: Determine the effect of mechanical stimuli on the phenotype of macrophages in vitro. Myeloid cells will be cultured and various, defined mechanical stimuli will be applied in a specialized chamber. The phenotypes of the cells will be compared by flow cytometry.
Aim 2: Define the role of the Ca2+ channel Piezo1 in myeloid mechanosensing and establish a reporter system for the quantification of Piezo1-mediated Ca2+ flux in vitro and in vivo. The impact of Piezo1 stimulation on cytoplasmic Ca2+ concentration will be assessed using specific inhibitors/activators in vitro. To quantify Piezo1-induced Ca2+ flux in the cytoplasm in vivo, we plan to use mice carrying a genetically encoded cytoplasmic calcium sensor in macrophages. We will relate the sensor signals to mechanical stimuli in vitro. Subsequently, we aim to employ this system to locate and quantify mechanically induced Ca2+ flux in myeloid cells by intravital FRET-FLIM microscopy,6 and correlate this signal to tissue dynamics, to map the pressure conditions in vivo.
Aim 3: Determine to what extent Piezo1-mediated mechanosensing in myeloid cells of the lamina propria contributes to the intestinal immune response in vivo. The phenotype of intestinal macrophages will be compared by flow cytometry and multiplexed histology in mice with a deficiency of Piezo1 in myeloid cells to wildtype mice. Initial analyses will be performed under homeostatic conditions. Subsequently, we will challenge the mice by infecting them with intestinal parasites, which are known to mechanically impact on the tissue, either by attaching to the intestinal wall or by inducing altered gut peristalsis.
References
- Pascual-Reguant A, Kohler R, Mothes R, Bauherr S, Uecker R, Holzwarth K, Kotsch K, Seidl M, Philipsen L, Müller W, Hernández D, Romagnani C, Niesner R, Hauser AE. Phenotypic and spatial characteristics of human Innate Lymphoid Cells revealed by highly multiplexed histology. Nat Commun. 2021. Accepted for publication.
- Stefanowski J, Lang A, Rauch A, Aulich L, Kohler M, Fiedler AF, Buttgereit F, Schmidt-Bleek K, Duda GN, Gaber T, Niesner RA, Hauser AE. Spatial Distribution of Macrophages During Callus Formation and Maturation Reveals Close Crosstalk Between Macrophages and Newly Forming Vessels. Front Immunol. 2019;10: 2588. doi: 10.3389/fimmu.2019.02588.
- Holzwarth K, Kohler R, Philipsen L, Tokoyoda K, Ladyhina V, Wahlby C, Niesner RA, Hauser AE. Multiplexed fluorescence microscopy reveals heterogeneity among stromal cells in mouse bone marrow sections. Cytometry A. 2018;93: 876-888. doi: 10.1002/cyto.a.23526.
- Solis AG, Bielecki P, Steach HR, Sharma L, Harman CCD, Yun S, de Zoete MR, Warnock JN, To SDF, York AG, Mack M, Schwartz MA, Dela Cruz CS, Palm NW, Jackson J, Flavell RA. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019; 573: 69-74. doi: 10.1038/s41586-019-1485-8.
- Lindquist RL, Bayat-Sarmadi J, Leben R, Niesner R, Hauser AE. NAD(P)H Oxidase Activity in the Small Intestine Is Predominantly Found in Enterocytes, Not Professional Phagocytes. Int J Mol Sciences. 2018; 19:1365. doi: 10.3390/ijms19051365.
- Ulbricht C, Leben R, Rakhymzhan A, Kirchhoff F, Nitschke L, Radbruch H, Niesner RA, Hauser AE. Intravital quantification of absolute cytoplasmic B cell calcium reveals dynamic signaling across B cell differentiation stages. Elife. 2020; 10:e56020. doi: 10.7554/eLife.56020.
Immunophenotyping in bullous pemphigoid to identify new therapeutic targets
Scientific interest within the context of the graduate college:
Our laboratory focuses on the immunological and molecular pathomechanisms of the skin with the aim to identify approaches for personalized and preventive medicine. One group of our translational research interests are chronic inflammatory autoimmune diseases of the skin. In addition to preclinical models and in vitro methods, we use skin and blood samples from patients for biomarker identification. We are particularly interested in specialized T cell responses and cytokine signaling pathways (JAK/STAT).
Project description:
Blistering autoimmune dermatoses (bAD) represent a heterogeneous group of skin diseases characterized by autoantibodies against structural proteins. In pemphigoid diseases, the formation of IgG and, more rarely, IgA/IgM antibodies against hemi desmosomes lead to loss of contact of the lower keratinocyte layer with the basal membrane. Subepidermal cleft formation occurs, resulting in the formation of blisters.1 The most common pemphigoid disease of old age is bullous pemphigoid (7/8 LD), followed by rarer variants such as lamin γ1 and scarring mucinous pemphigoid. While B cells are primarily responsible for autoantibody production, T cells appear to play an important role in disease development.
In recent work on the pathogenesis of bAD, we were able to investigate the relationship between specialized T follicular helper (Tfh) cells and autoreactive B cells for the production of autoantibodies in bAD by comprehensive analyses of the immune system (RNA-seq, immunoprofiling, T/B cell cultures).2 In bullous pemphigoid, the risk of disease increases with age without knowing the immunological precursors for this. In this project, we aim to clarify whether specialized Tfh cells and their cytokines differ in healthy individuals and bAD patients in an age-dependent manner. The aim is to investigate their influence on the pathogenesis of the disease and to identify new markers to predict the risk for the manifestation of bAD or even to use them preventively.3
In this project, Tfh populations of peripheral blood will be studied by multiparametric flow cytometry. We are particularly interested in the differences between healthy and bAD patients and the changes in different age decades. Furthermore, the cytokine profile (IL-4, IL-17, IL-21, IL-6, IFN-y) will be investigated.4 These results will be compared on the one hand with antibody titers against BP180 and BP230 and on the other hand with transcriptome analyses of healthy and lesional skin (bAD). The goal of the PhD thesis is to determine disease-associated patterns relevant for early detection or risk of manifestation of bAD, by evaluating the Tfh populations, cytokine profile, autoantibody titers, and age-related parameters.
References
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019; 394(10201):882-894. doi: 10.1016/S0140-6736(19)31778-7.
- Holstein J, Solimani F, Baum C, […], Pfützner W, Ghoreschi K, Möbs C. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J Allergy Clin Immunol. 2020: S0091-6749(20)31624-9. doi: 10.1016/j.jaci.2020.11.008. Epub ahead of print.
- Eckardt J, Eberle FC, Ghoreschi K. Diagnostic value of autoantibody titres in patients with bullous pemphigoid. Eur J Dermatol. 2018; 28(1):3-12. doi: 10.1684/ejd.2017.3166.
- Li Q, Liu Z, Dang E, […], Yang L, Shi X, Wang G. Follicular helper T Cells (Tfh) and IL-21 involvement in the pathogenesis of bullous pemphigoid. PLoS One. 2013; 8(7):e68145. doi: 10.1371/journal.pone.0068145
Group 3 Innate Lymphoid Cells as regulators of intestinal organ hypertrophy in response to increased metabolic demands during pregnancy and lactation
Scientific interest within the context of the graduate college:
We study development and function of the innate immune system, in particular of innate lymphoid cells (ILC). A current focus is to obtain a molecular understanding of how the innate immune system, by integrating environmental signals, contributes to tissue physiology and health. Recent studies have revealed ever more intriguing relationships between innate immune system components and basic developmental and biologic processes that are likely to reveal unsuspected pathways by which the immune system might be plumbed to improve health and health span. These lines of research have suggested new functions of the immune system for processes such as tissue homeostasis, morphogenesis, metabolism, regeneration and growth. Our research is developing by crossing boundaries of disciplines (immunology, microbiology, developmental biology, stem cell biology, nutrition sciences, tumor biology, regenerative medicine etc.) and is, by nature, highly interdisciplinary.
Project description:
Based on our data, we hypothesize that ILC3 play an important role in directly instructing adaptation of the intestinal organ to changing metabolic needs by affecting programs in epithelial stem cells or their immediate progeny. Available data has interrogated the role of ILC3: stem cell modules in the context of intestinal damage. We wondered if ILC3 are involved in more physiological adaptative processes. One of the biggest challenges to metabolic demands in life is pregnancy. During gestation and lactation, the female organism undergoes major physiological changes to accommodate the developing offspring prominent among them considerable growth of the crypt-villus axis of the small intestine. We have recently developed a sophisticated method to record crypt-villus length and noted that mice lacking ILC3 (i.e., Rorc(gt)Gfp/Gfp mice) do not show pregnancy and lactation-induced epithelial hypertrophy. Interestingly, such absence of intestinal growth led to reduced caloric absorption by enterocytes and reduced caloric content of breast milk.
We hypothesize that ILC3 act on intestinal stem cells enhancing differentiation of enterocytes for increased nutrient absorption. Our research may provide a novel conceptual framework of how tissue and metabolic adaptation can be sustained by ILC3 that may dynamically adjust epithelial cell function to a variety of physiological demands.
Our major goals are (1) to define the role of ILC3 in pregnancy and lactation-induced epithelial hypertrophy on a molecular level, (2) to analyze the impact of ILC3 on stem cell representation, niche population dynamics using stochastic multi-color fate labelling, and (3) to understand how ILC3 regulate epithelial cell metabolism and tissue growth during pregnancy and lactation.
References
- Guendel F, Kofoed-Branzk M, Gronke K, […], Mashreghi MF, Kruglov AA, Diefenbach A. Group 3 innate lymphoid cells program a distinct subset of IL-22BP-producing dendritic cells demarcating solitary intestinal lymphoid tissues. Immunity. 2020; 53:1015-1032. doi: 10.1016/j.immuni.2020.10.012.
- Schaupp L, Muth S, Rogell L, […],Schild H, Diefenbach A1,*,Probst HC*. Microbiota-induced tonic type I interferons instruct a poised basal state of dendritic cells. Cell. 2020; 181:1080-1096. doi: 10.1016/j.cell.2020.04.022. 1Lead Senior Author; *equal contribution.
- Gronke K, Hernández PP, Zimmermann J, […], Glatt H, Triantafyllopoulou A, Diefenbach A. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019; 566:249-253. doi: 10.1038/s41586-019-0899-7.
- Klose CSN, Flach M, Möhle L, […], Dunay IR, Tanriver Y, Diefenbach A. Differentiation of type 1 ILCs from a common progenitor to helper-like innate lymhoid cell lineages. Cell. 2014; 157:340-356. doi: 10.1016/j.cell.2014.03.030.
- Klose CSN, Kiss EA, Schwierzeck V, […], Waisman A, Tanriver Y, Diefenbach A. A T-bet gradient controls the fate and function of CCR6– RORγt+ innate lymphoid cells. Nature. 2013, 494:261-265. doi: 10.1038/nature11813.
Impact of the microbiome and the immune system on cardiovascular risk in chronic kidney disease
Scientific interest within the context of the graduate college:
Our laboratory investigates the interaction of dietary factors with the gut microbiota and the host, especially with the host’s immune system, in the context of cardiovascular and renal disease.1-4 Arterial hypertension and chronic kidney disease (CKD) are of particular interest, as both conditions are associated with a significantly increased cardiovascular risk. We combine experimental model systems with exploratory clinical studies to better understand the microbiota-host interaction. We aim to use our findings to further define health in this context and to better understand transitions to disease.
CKD is associated with dysregulated immune responses5 and high cardiovascular risk.6 In addition, CKD is associated with changes in the composition and function of the microbiota. Indole metabolites of bacterial origin are of particular interest here, as they can accumulate in the blood with declining renal function and have an immunomodulatory potential.3 It is unclear to what extent these CKD-associated microbial changes contribute to cardiovascular risk. To investigate this question, microbiota from CKD patients, as well as from CKD mouse models and healthy control groups will be transplanted into germ-free mice. In the recipients, immunological changes and cardiovascular parameters will be quantified and the molecular mechanisms involved will be investigated in more detail.
Project description:
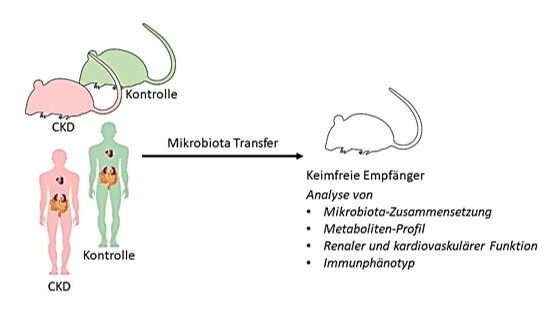
WP 1: Microbiota transfer from CKD mouse models. Fecal samples from different CKD animal models (incl. healthy controls) will be analyzed (composition, metabolites) and used to colonize germ-free mice. The recipients will then be phenotyped in regard to their immune function (isolation of immune cells from different end organs and analysis by flow cytometry) and cardiovascular function (vascular and cardiac function, intestinal barrier, etc.) and compared with parameters of the donors.
WP2: Microbiota transfer of human-associated microbiota. Stool samples from CKD patients and healthy controls will be collected, analyzed by sequencing (composition, metabolites), and transplanted into germ-free mice (Fig. 1). Immunological, microbial, and cardiovascular parameters will be analyzed between the two groups of recipients and the extent to which human CKD-associated pathologies are transferable to the mouse model will be investigated.
References
- Bartolomaeus H, Balogh A, Yakoub M, […], Muller DN, Stegbauer J, Wilck N. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019; 139:1407-1421. doi: 10.1161/CIRCULATIONAHA.118.036652.
- Wilck N, Balogh A, Marko L, Bartolomaeus H and Muller DN. The role of sodium in modulating immune cell function. Nat Rev Nephrol. 2019; 15:546-558. doi: 10.1038/s41581-019-0167-y.
- Wilck N, Matus MG, Kearney SM, […], Linker RA, Alm EJ, Muller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017; 551:585-589. doi: 10.1038/nature24628.
- Bartolomaeus H, Avery EG, Bartolomaeus TUP, […], Wilck N, Kushugulova A, Forslund SK. Blood Pressure Changes Correlate with Short-Chain Fatty Acids Production Shifts Under a Synbiotic Intervention. Cardiovasc Res. 2020; 116:1252-1253. doi: 10.1093/cvr/cvaa083.
- Sato Y, Yanagita M. Immunology of the ageing kidney. Nat Rev Nephrol. 2019; 15:625-640. doi: 10.1038/s41581-019-0185-9.
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004; 351:1296-305. doi: 10.1056/NEJMoa041031.
NKp46+ ILC control inflammatory responses in lupus nephritis
Scientific interest within the context of the graduate college:
Our research aims to understand the role of tissue-resident cells of the innate immune system in the prevention of chronic inflammatory diseases such as systemic lupus erythematosus and inflammatory bowel disease. Our goal is to identify mechanisms that may inhibit the transition from homeostasis to chronic inflammatory disease and to determine the role of tissue-resident cells of the innate immune system in this process. Understanding such mechanisms may allow to answer the question of why some patients are susceptible to chronic autoimmune-related inflammatory diseases and others are not, and how to improve/achieve resistance to chronic inflammatory diseases.
Project description:
In Systemic Lupus Erythematosus (SLE), autoantibodies and immune complex deposition are required for pathogenesis however they are not sufficient to cause tissue damage. Tissue-specific cellular regulators that maintain tissue homeostasis and protect against inflammatory responses are largely unknown. Our overall aim in this project was to investigate the mechanisms of tissue resilience and the transition to the onset of autoimmune-induced tissue damage in the kidney. The investigated signaling pathways could reveal new therapeutic targets for patients who have already developed autoantibodies.
Characterization of the immune microenvironment in gastrointestinal tumors using single-cell techniques
Student

Principle Investigator
Principle Investigator

Scientific interest within the context of the graduate college:
Our lab studies the role of immune cells and inflammatory processes in the liver. Infiltration and activation of immune cells play an important role during the development of acute and chronic liver diseases, but the exact molecular and cellular mechanisms leading to the development of liver inflammation have not been fully elucidated until now. We are exploring the inflammatory processes during acute liver failure, non-alcoholic fatty liver disease and steatohepatitis (NASH), liver cirrhosis and liver cancer in order to develop new diagnostic and therapeutic strategies. Furthermore, a better understanding of how both pro- and anti-inflammatory pathways can disrupt the homeostatic processes of a healthy liver is critical for the prevention of liver diseases in the first place.
During homeostasis, the liver plays an important role in adaptation to environmental influences as it is constantly exposed to antigens from the gastrointestinal system, and plays a critical role in maintaining a balance between tolerance to harmless antigens (eg. food proteins or commensal bacteria) and control of pathogens.1 When this balance is disrupted (termed maladaptation) the resulting immune-mediated changes can lead to chronic liver diseases and ultimately cancer.
Project description:
We are especially interested in characterizing the microenvironment of gastrointestinal tumors because these tumors usually develop under chronic inflammatory conditions but then often switch to an immunosuppressive environment.2 This makes them insusceptible to many therapeutic strategies including immunotherapies, which are emerging as new treatment strategies for many types of cancer.3,4 In order to better understand how this switch happens and how the immune-microenvironment in the tumor can be influenced by the surrounding tissue and vice versa, this project aims at investigating the immune phenotype of gastrointestinal tumors. Tumor samples from patients with gastrointestinal tumors (eg. hepatocellular carcinoma, neuroendocrine tumors), as well as chronic liver diseases (eg. NASH, NAFLD), will be analyzed using high throughput single-cell technologies, including single-cell RNA-sequencing, spectral flow cytometry, and multiplex immunohistochemistry. All techniques are already set up in our lab5,6 and will be used to analyze samples already available in a biobank in our department as well as prospectively acquired tissue samples obtained during surgery (resections, explants). Characterization of the peri-/intratumoral immune cell populations and correlation with clinical data (underlying etiology, medical history, therapy response, survival time etc.) will contribute to our understanding of the immune microenvironment in gastrointestinal tumors and how this influences disease outcome. These data will be critical to developing novel strategies to prevent chronic inflammatory liver diseases and tumor formation as well as guide the development of personalized therapeutic strategies and future clinical trials.
References
- Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol. 2018; 36: 247-277. doi: 10.1146/annurev-immunol-051116-052415.
- Rohr-Udilova N, Klinglmüller F, Schulte-Hermann R, […], Jensen-Jarolim E, Eferl R, Trauner M. Deviations of the immune cell landscape between healthy liver and hepatocellular carcinoma. Sci Rep. 2018; 8:6220. doi: 10.1038/s41598-018-24437-5.
- Zhu AX, Finn RS, Edeline J, […], Siegel AB, Cheng AL, Kudo M, KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018; 19: 940-952. doi: 10.1016/S1470-2045(18)30351-6.
- Brahmer JR, Tykodi SS, Chow LQM, […], Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012; 366:2455-2465., doi: 10.1056/NEJMoa1200694.
- Ramachandran P, Dobie R, Wilson-Kanamori JR, […], Marioni JC, Teichmann SA, Henderson NC. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019; 575:512-518. doi: 10.1038/s41586-019-1631-3.
- Guillot A, Kohlhepp MS, Bruneau A, Heymann F, Tacke F. Deciphering the Immune Microenvironment on A Single Archival Formalin-Fixed Paraffin-Embedded Tissue Section by An Immediately Implementable Multiplex Fluorescence Immunostaining Protocol. Cancers (Basel). 2020; 12:2449. doi: 10.3390/cancers12092449..
From infection to fibrosis – defining common immune determinants of disease and resolution
Project description:
Resilience to infections is equally defined by the host’s ability to clear the infecting pathogen and to resolve and mitigate secondary injury, and thus to restore and maintain tissue homeostasis. These physiological processes of resolution and repair, however, intersect with pathogenic responses in chronic organ fibrosis. The role of viral infections in fibrotic diseases are not well defined. We have recently identified a population of pulmonary macrophages that arises in patients with severe COVID-19 and fibroproliferative ARDS. These macrophages share core features with profibrotic macrophages in idiopathic pulmonary fibrosis or liver cirrhosis. Here we aim to dissect the signals that program profibrotic monocyte and macrophage responses in acute infections and chronic fibrosis in order to define checkpoints and potential therapeutic targets, with the aim of rewiring responses to tissue injury and restoring tissue homeostasis.
Membrane-bound neutrophil elastase as a potential biomarker for early detection of chronic inflammatory diseases
Scientific interest within the context of the graduate college:
Biomarkers play a central role in detecting chronic inflammatory diseases before irreversible tissue damage occurs. These measurable, ideally disease-specific, indicators can be detected in the organism even before the onset of symptoms. For example, neutrophil elastase (NE), a serine protease secreted by activated neutrophils and essential for the innate immune response to pathogen infections, already serves as a biomarker for certain chronic inflammatory diseases of the respiratory and digestive tracts. In these cases, the activity of secreted, i.e. “free” NE, which is released during chronic inflammatory processes and damages tissues due to proteolytic activity, has been determined so far. In previous work, we have shown that NE also occurs membrane-bound on the surface of activated neutrophils in the airways of chronic inflammatory lung diseases such as cystic fibrosis. There, membrane-bound NE is already catalytically active long before the activity of free NE can be detected and it is involved in airway damage.1,2 The results of these studies suggest that determining the activity of membrane-bound NE could also enable earlier diagnosis of other chronic inflammatory diseases and facilitate personalized therapy.
Project description:
The aim of this translational research project is to investigate the role of membrane-bound NE activity on neutrophils circulating in the blood of healthy subjects and patients with chronic inflammatory (autoimmune) diseases in childhood and thus establish a method for early disease detection. For this purpose, a reporter molecule based on Förster resonance energy transfer (FRET) technology we developed is used.3 This allows the detection of NE activity on the surface of living, freshly isolated cells ex vivo using flow cytometry. In addition to state of the artcytometry and cell biology technologies, this experimental MD thesis also applies basic molecular biology laboratory work and microscopy and correlates experimental data with clinical patient data on disease progression.
References
- Gehrig S, Duerr J, Weitnauer M, […], Dalpke AH, Schultz C, Mall MA. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am J Respir Crit Care Med. 2014; 189:1082-1092. doi: 10.1164/rccm.201311-1932OC.
- Dittrich AS, Kühbander I, Gehrig S, […], Herth F, Schultz C, Mall MA. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis.Eur Respir J. 2018; 51:1701910. doi: 10.1183/13993003.01910-2017.
- Hagner M, Frey DL, Guerra M, […], Herth FJF, Schultz C, Mall MA. New method for rapid and dynamic quantification of elastase activity on sputum neutrophils from patients with cystic fibrosis using flow cytometry. Eur Respir J. 2020; 55:1902355. doi: 10.1183/13993003.02355-2019.
The adaptation of the intestinal epithelium to an oxalate-containing diet
Student

Principle Investigator

PD Dr. Martin Reichel
Dr. Nicola Wilck
Scientific interest within the context of the graduate college:
Our laboratory focuses on the mechanisms involved in maintaining oxalate homeostasis. Oxalate is a component of various foods, found in different vegetables, nuts, but also in tea and coffee. High urinary oxalate concentrations lead to kidney stones, the second most common kidney disease after hypertension. Oxalate represents the most common component of kidney stones. When kidney function is reduced as part of chronic kidney disease, for example as a result of diabetes or hypertension, oxalate concentrations in the blood also increase. This is associated with various organ damage and increased cardiovascular mortality (manuscript in revision).
Our research group has cloned the first oxalate transporter (SLC26A6).1 SLC26A6 is expressed in different organs. The transporter is located on the apical side of epithelia and actively secretes oxalate into the intestinal lumen2 and urine.3,4 Via this transport process, the oxalate concentration in the body is kept low. In the absence of the transporter, there is increased uptake of oxalate from the intestine and consequent formation of kidney stones5 and progressive kidney damage.6 Several research groups have also shown that oxalate can activate immune cells.7 Recently, we demonstrated that the oxalate transporter SLC26A6 is widely expressed in immune cells (unpublished data).
Project description:
The subject of the investigation will be the question of what influence an oxalate-containing diet has on the intestinal epithelium and what role immune cells and SLC26A6 play in the recognition of dietary oxalate. Our hypothesis is that dietary oxalate modifies the intestinal epithelial composition to enhance the shift of cytoplasmic SLC26A6 to the epithelium membrane and this process may depend on recognition of oxalate by immune cells.
WP 1: Characterization of the intestinal epithelium during an oxalate-containing diet. Two groups of mice are compared. One group receives an oxalate-free diet; a second group contains an oxalate-containing diet. After three weeks, both groups are analyzed for the following parameters: (i) microbiome composition, (ii) intestinal epithelium characterization (absorptive/secretory cells), (iii) SLC26A6 transporter expression (absorptive/secretory cells).
WP 2: Immunophenotyping of the intestine after an oxalate diet. The same two groups of mice as in WP 1 and additionally SLC26A6-/- mice will be examined for resident and migrated immune cells in (i) intestine and (ii) kidney.
References
- Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A. 2001; 98:9425-9430. doi: 10.1073/pnas.141241098.
- Neumeier LI, Thomson RB, Reichel M, Eckardt KU, Aronson PS, Knauf F. Enteric Oxalate Secretion Mediated by Slc26a6 Defends against Hyperoxalemia in Murine Models of Chronic Kidney Disease. J Am Soc Nephrol. 2020. 31:1987-1995. doi: 10.1681/ASN.2020010105.
- Knauf F, Ko N, Jiang Z, […], Van Ittalie CM, Anderson JM, Aronson PS. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J Am Soc Nephrol. 2011; 22:2247-2255. doi: 10.1681/ASN.2011040433.
- Knauf F, Thomson RB, Heneghan JF, […], Egan ME, Alper SL, Aronson PS. Loss of Cystic Fibrosis Transmembrane Regulator Impairs Intestinal Oxalate Secretion. J Am Soc Nephrol. 2017; 28:242-249. doi: 10.1681/ASN.2016030279.
- Jiang Z, Asplin JR, Evan AP, […], Nottoli TP, Binder HJ, Aronson PS. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet. 2006; 38:474-478. doi: 10.1038/ng1762.
- Knauf F, Asplin JR, Granja I, […], David RJ, Flavell RA, Aronson PS. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 2013; 84:895-901. doi: 10.1038/ki.2013.207.
- Knauf F, Brewer JR, Flavell RA. Immunity, microbiota and kidney disease. Nat Rev Nephrol. 2019; 15:263-274. doi: 10.1038/s41581-019-0118-7.
Congenital group 2 lymphocytes are a major source of interleukin 5, essential for development and function of B1 cells
Scientific interest within the context of the graduate college:
Type 2 immune responses promote tissue homeostasis as well as tissue remodeling and protect against infections with macroparasites but can become detrimental when triggered against non-infectious environmental stimuli1. The cytokines IL-25, IL-33, and TSLP are strong activators of type 2 inflammation in tissues via stimulation of group 2 innate lymphoid cells (ILC2s) and other innate immune cells, such as eosinophils, mast cells, basophils, and alternatively activated macrophages resulting in a cytokine milieu, which promotes differentiation of T helper 2 cells and secretion of immunoglobulin E.1,2 Although ILC2s become quickly activated, the precise role in orchestrating type 2 immune responses remains elusive due to the limitations in specifically targeting this population in the presence of adaptive immune cells because of the large overlap in expression of ILC2s with T cells and other immune cells.
Project description:
To guide over this major limitation in the field we could recently generate and evaluate a model to specifically deplete ILC2s. Exploiting the possibilities of this newly generated tool, this proposal now aims to systematically investigate immunoregulation mediated by ILC2s at mucosal surfaces of the gastrointestinal tract. Using a combination of immunophenotyping, state-of-the-art sequencing technology, and microbiome analysis we will explore how the adaptive immune response, such as affinity maturation of immunoglobulins is affected in the absence of ILC2s.3,4 The signal circuits involving ILC2-mediated regulation of microbiota either directly or indirectly via regulation of immunoglobulins and B cell responses will be investigated as well. Our working hypothesis predicts that ILC2s determine the microbiota colonization in early life through different pathways including but not limited to affinity maturation of antibodies. The models to test this hypothesis are available in our lab and the techniques are carried out on a daily basis without the requirement to establish novel methods from scratch.
Delineating the regulation of type 2 immune responses by ILC2s will be key to understand how type 2 immune responses are orchestrated. Using both focused and global experimental approaches our research has the potential to discover novel molecular pathways, which can be harnessed for the maintenance of homeostasis and prevention of chronic inflammation.
References
- Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012; 484:465-472. doi: 10.1038/nature11047.
- Klose CSN, Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020; 30:475-491. doi: 10.1038/s41422-020-0323-8.
- Moro K, Yamada T, Tanabe M, […], Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010; 463:540-544. doi: 10.1038/nature08636.
- Nussbaum JC, Van Dyken SJ, von Moltke J, […], Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013; 502:245-248. doi: 10.1038/nature12526.


























