The role of the epithelial transcription factor NF-κB in homeostasis, development and regeneration of chronic inflammatory bowel diseases
Scientific interest within the context of the graduate college:
Our group “Signal Transduction in Health and Disease” (Department of Gastroenterology and Hepatology, Charité Virchow) aims to understand the molecular mechanisms involved in tissue homeostasis, inflammation, and resolution of inflammation. Our main focus is on the transcription factor NF-κB and its role in the intestinal epithelium. Recent studies showed that crosstalk between epithelium and immune cells changes in health and disease, and that is in part due to altered functions of NF-κB. Our group aims to decipher that crosstalk and identify the changes. Our research is highly interdisciplinary and spans different fields including biochemistry, immunology, stem cell biology, and cancer biology.
Project description:
NF-κB is deemed the master regulator of pro-inflammatory response and its activation directly correlates with severity of inflammation in Inflammatory Bowel Disease (IBD).1 Although in immune cells, NF-κB plays largely pro-inflammatory role, in intestinal epithelium its function is poorly understood. Many IBD therapies rely on suppression of NF-κB. These therapies however inhibit NF-κB across different tissues and cell types, including healthy ones. This can lead to many adverse effects, including susceptibility to infection and carcinogenesis. We have recently shown that a single stress stimulus sequentially activates functionally distinct transcriptomes of NF-κB: anti-apoptotic and pro-inflammatory.2-3 We also showed that in homeostasis, NF-κB directs differentiation of stem cells.4 To identify therapies that specifically target only the pro-inflammatory NF-κB, it is critical to decipher what roles NF-κB plays in health, in acute inflammation, and finally in mucosal healing.
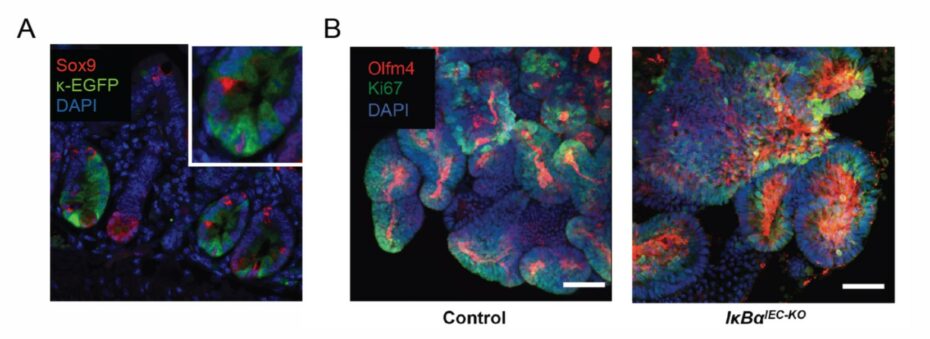
Based on our recently published and unpublished data we propose that acute, immediate activation of NF-κB in intestinal epithelium is necessary for resolution and for regaining homeostasis. To address this hypothesis we will use single-cell datasets, intestinal organoids, and transgenic mouse models. We have recently established transgenic mice that provide a specific readout of NF-kB activity as well as mice with either specific suppression or constitutive activation of NF-kB in intestinal epithelium.2-4 These tools could enable us, for the first time, to determine where and how NF-κB can be targeted to both prevent chronic inflammation or to promote resolution.
Our key aims are:
1) To identify which cells activate NF-kB under homeostasis versus in inflammation, and to determine which transcriptional programs NF-kB initiates in these cells.
2) To determine how repression or constitutive activation of NF-kB affects intestinal homeostasis, acute inflammation, and resolution.
3) Identify which singling pathways can be targeted to safeguard homeostasis or promote resolution of inflammation.
References
- Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008; 263:591-596. doi: 10.1111/j.1365-2796.2008.01953.x.
- Kolesnichenko M, Mikuda N, Höpken UE, […], Schmidt-Ullrich R, Schmitt CA, Scheidereit C. Transcriptional repression of NFKBIA triggers constitutive IKK- and proteasome-independent p65/RelA activation in senescence. EMBO J. 2021; 40:e104296. doi: 10.15252/embj.2019104296.
- Mikuda N, Schmidt-Ullrich R, Kärgel E, […], Scheidereit C, Kühl AA, Kolesnichenko M. Deficiency in IκBα in the intestinal epithelium leads to spontaneous inflammation and mediates apoptosis in the gut. J Pathol. 2020; 251:160-174. doi: 10.1002/path.5437.
- Brischetto C, Krieger K, Klotz C, […], Heuberger J, Scheidereit C, Schmidt-Ullrich R. NF-κB determines Paneth versus goblet cell fate decision in the small intestine. Development. 2021; 148:dev199683. doi: 10.1242/dev.199683.

