Disease phenotypes in Crohn’s disease – Does the key lie in the mucosal microenvironment?
Principal Investigator

Dr. Lea-Maxie Haag
Dr. Malte Lehmann
Scientific interest within the context of the graduate college:
Based at the Department of Gastroenterology, Infectious Diseases and Rheumatology at Campus Benjamin Franklin, Charité – Universitätsmedizin Berlin, our main clinical and research focus are inflammatory bowel diseases (IBD). Crohn’s disease (CD), one of the primary forms of IBD, exhibits various clinical phenotypes, including inflammatory (B1), stricturing (B2), and fistulizing (B3) disease phenotypes. We aim at understanding the biological differences between these phenotypes at the level of the mucosal microenvironment. With our large endoscopy unit and outpatient clinic, as well as gastroenterology ward, we have access to biological samples like intestinal biopsies along with the corresponding clinical data.
Project description:
What is the underlying mechanism and biology leading to the different disease phenotypes in Crohn’s disease?
Within this project, we aim to better understand the mechanisms responsible for the development of the different disease phenotypes in CD. We aim to analyze the intestinal microenvironment on a single-cell level by metabolic imaging mass cytometry. This enables us to investigate activity and metabolic states of intestinal structural as well as innate and adaptive immune cells. Moreover, we will focus on the relevance of cell-cell interactions and cellular neighborhoods, which define but are also common to distinct disease phenotypes (Figure 1). The proposed project will be associated with the clinical study ‘InFlame’ (DRKS00031203), which is already running since early 2023. All protocols and methods are established and running in the lab and have been approved by the ethics board of the Charité – Universitätsmedizin Berlin. The existing study team and the ongoing recruitment process guarantee intensive support and the provision of all necessary infrastructure.
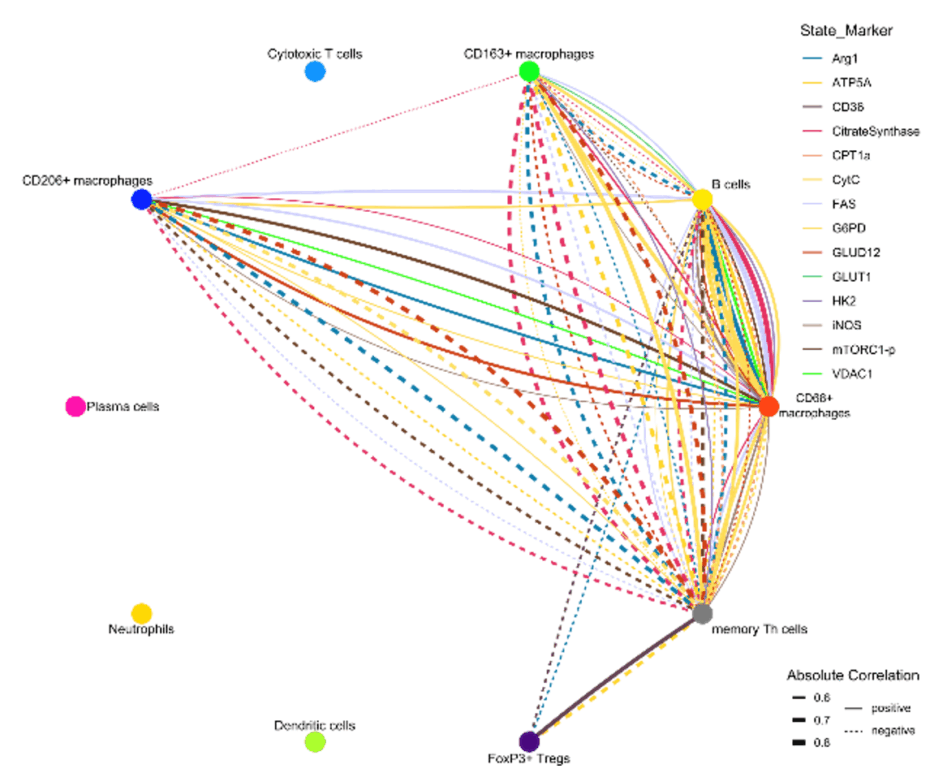
Figure 1. Iconography of correlations of state marker expression to the distance between immune cell clusters. Only correlations with r > 0.5 or < 0.5 and p < 0.05 are shown. The color of the lines indicate the respective state marker, the width of the line the absolute value of correlation. Straight lines indicate positive correlations, doted lines negative correlations.
WP1. Crohn’s Disease Cohort Recruitment. Completing the B1-B2-B3 Crohn’s disease cohort (recruitment already ongoing).
WP2. Imaging Mass Cytometry of Intestinal Tissue to Study Crohn’s Disease. Performing imaging mass cytometry on intestinal tissue (using Formalin fixed paraffin embedded biopsies) and analyses of the different disease phenotypes in Crohn’s disease.
References
- Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013; 62(7):1072-1084.
- Rieder F, Mukherjee PK, Massey WJ, Wang Y, Fiocchi C. Fibrosis in IBD: from pathogenesis to therapeutic targets. Gut. 2024; 73(5):854-866.
- Lehmann M, Weixler B, Elezkurtaj S, Loddenkemper C; TRR241 IBDome Consortium; Kühl AA, Siegmund B. Spatial Single Cell Profiling Using Imaging Mass Cytometry: Inflammatory Versus Penetrating Crohn’s Disease. J Crohns Colitis. 2024; 18(8):1305-1318.
- Lehmann M, Allers K, Heldt C, Meinhardt J, Schmidt F, Rodriguez-Sillke Y, […], Weidinger C, Kühl AA, Siegmund B. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8+ T cells. Mucosal Immunol. 2021; 14(6):1381-1392.
- Avery EG*, Haag LM*, McParland V, Kedziora SM, Zigra GJ, Valdes DS, […], Siegmund B, Wiig H, Müller DN. Intestinal interstitial fluid isolation provides novel insight into the human host-microbiome interface. Cardiovasc Res. 2025; 121(5):803-816. *equal contribution
- Stankey CT*, Bourges C*, Haag LM*, Turner-Stokes T, Piedade AP, Palmer-Jones C, […], Wallace C, Thomas DC, Lee JC. A disease-associated gene desert directs macrophage inflammation through ETS2. Nature. 2024; 630(8016):447-456. *equal contribution