Identification of a gut microbiota signature in patients with drug-resistant epilepsy upon ketogenic diet treatment
Principal Investigator

Dr. Laura Díaz Marugán
Scientific interest within the context of the graduate college:
This project aims to uncover the intricate relationship between diet, gut microbes, and drug-resistant epilepsy (DRE). Focusing on the ketogenic diet (KD), the research seeks to identify specific gut bacteria associated with improved brain health in KD responders. The objectives include investigating the bacterial signature in a large cohort of KD responder and non-responder people, isolating potentially beneficial bacteria, and studying their metabolic functions. The study aims to understand how the gut microbiota influences the success of KD treatment, potentially serving as a biomarker for treatment efficacy. By bridging the gap between diet, gut microbes, and neurological conditions, this research may pave the way for personalized interventions in managing drug-resistant epilepsy.
This project stands out for its strong clinical and experimental approach in close collaboration with Prof. Dr. Angela Kaindl from the Clinic for Pediatrics and Neurology, Charité. The successful candidate will benefit from a training in which he/she will be able to put into practice his/her knowledge of the medical field and learn concepts, techniques and strategies of experimental research in the laboratory, paving the way to better understand how we could modulate the course of epilepsy via microbiota-diet modulation.
In this research project, the successful candidate will be able to:
- Manage human sampling collection for research purposes.
- Learn about the ketogenic diet as a therapy in the clinics.
- Learn about patient’s group stratification according to the different profiles in response to the treatment.
- Prepare and analyse questionnaires to track dietary habits, life-style habits and quality of life.
- Extract and quantify genomic DNA from human stools.
- Culture bacteria from human stools and characterize them via full genome sequence.
- Get knowledge in microbial composition bioinformatic analysis.
- Process serum samples for proteomic analysis.
Project description:
Epidemiology and social impact of epilepsy and drug-resistant epilepsy (DRE). Epilepsy is one of the most common neurological disorders, affecting more than 50 million people worldwide and 6 million people in Europe (Singh & Sander, 2020; Sirven, 2015). It affects people of all ages and is characterized by epileptic seizures (Berg et al., 1999; Fiest et al., 2017; Guerrini, 2006; Sirven, 2015). Uncontrolled seizures can lead to developmental delay, cognitive deficits, memory and learning difficulties. They can also cause sudden unexpected death in people with epilepsy (SUDEP) (Sperling, 2004). Current treatments include anti-seizure medicines (ASM), diet, epilepsy surgery and stimulation devices. ASM can control seizures in about two-thirds of people with epilepsy. However, one-third of patients with epilepsy cannot be controlled by two or more correctly selected and dosed ASM, what is called drug-resistant epilepsy (DRE) (Kwan et al., 2009, 2011; Picot et al., 2008; Sultana et al., 2021).
The microbiota-gut-brain axis: a modulator for epileptogenesis. The causes of epilepsy include structural, genetic, infectious, metabolic and immune factors (Guerri et al., 2020; Matin et al., 2015; Vezzani et al., 2016; Oliver et al., 2023; Rho & Boison, 2022). The gut-brain axis (GBA) has also been implicated in this respect (Iannone et al., 2019). Gut microbiota produces a variety of compounds that can affect brain function, such as neurotransmitters and metabolites, like short-chain fatty acids (SCFAs) (Chen et al., 2021; Dalile et al., 2019; Silva et al., 2020), some of which have shown anticonvulsant effects in rodents (De Caro et al., 2019; Mu et al., 2022; Olson et al., 2018). In patients, studies have confirmed that bacterial-based approaches, such as probiotics or microbiota-modifying treatments, can reduce seizure frequency. Indeed, the pilot usage of a mixture of probiotics (eight bacterial subspecies of Lactobacillus, Bacteroides and Streptococcus) reduced seizure frequency and improved the quality of life in patients with DRE (Gómez-Eguílaz et al., 2018). Similarly, fecal microbiota transplantation (FMT) for the treatment of Crohn’s disease (CD) symptoms ameliorated seizures frequency in a 22-year-old woman with a 17-year history of epilepsy, who stopped taking ASM after the FMT (He et al., 2017). Despite these promising results, the mechanisms by which the microbiota may affect the development of seizures in patients are completely unclear.
Ketogenic diet in the management of DRE. Alternative treatments for DRE include dietary treatments such as the ketogenic diet (KD) (Hemingway et al., 2001; Lyons et al., 2020). KD is a diet based on a high fat intake accompanied by moderate protein, and very low or no carbohydrate content, usually in a 3:1 or 4:1 ratio of caloric intake from lipids to both protein and carbohydrate together, and with a normal total energy intake. Restricting carbohydrate intake forces the metabolism to replace the preferred energy source, glucose, with fatty acids, which are oxidized in the liver to form ketone bodies. Although the classical KD has been used in epilepsy for many years and its efficacy in reducing seizures has been confirmed in several studies, especially when introduced in young age (Martin-McGill et al., 2020), the mechanism behind its action is not well understood in humans. In particular, it is not known why this dietary intervention has an anti-seizure effect in a proportion of the patients, while in others there is poor or no effect (Martin-McGill et al., 2020) or even worsening of seizure frequency or appearance of adverse effects (Cai et al., 2017; Newmaster et al., 2022; Yan et al., 2018). Although the exact mechanism is not yet known, several research studies in both mice and human have shown a link between the gut microbiota and the effects of the KD, topic that we recently discussed (Díaz-Marugan et al., 2024).
Hypothesis. Changes in the gut microbial composition by dietary intervention have a significant impact on the CNS function during the development of pathological conditions, such as seizures, via metabolites or immune mediators.
Therefore, this proposal aims to provide unprecedented insights into the molecular characteristics of commensal microbes after ketogenic diet that determine seizure improvement or worsening in different patients.
To achieve the overall aim, I have defined two objectives:
- Determine the (i) faecal microbiota composition and (ii) stool and blood metabolomic profile of children with DRE and treated with KD or with a non-KD diet compared to healthy children.
- Isolate and characterise the metabolic functions of commensal bacteria that thrive in children with DRE and treated with KD and show seizure improvement, no effect on seizures or worsening after KD introduction.
The successful candidate for this fellowship will focus on addressing Aim 1 and 2.
Aim 1: Human KD-associated microbiota and metabolomics. Hypothesis: In humans, the KD is associated with an overgrowth of gut bacteria capable of secreting neuroprotective metabolites with an anti-seizure activity. Aim: To investigate the (i) faecal microbiota composition and (ii) stool and blood metabolomic profile of children with drug-resistant epilepsy and treated with KD or with a non-KD diet, compared to healthy children. Work plan (WP): To address this first aim, we will recruit the following participants: (i) with drug-resistant epilepsy (DRE) for which KD is recommended. They will start the treatment with KD within an inpatient study (KD) (group 1), (ii) with DRE and non-KD diet (group 2), (iii) healthy children on a non-KD diet (group 3).
From group 1, several faecal samples, blood samples and questionnaire responses are collected. From group 2, a faecal sample, a blood sample and answers to a questionnaire are collected. A faecal sample and questionnaire responses will be collected from group 3. There will be 50 participants recruited in each group (within each group there will be a 1:1 male to female ratio).
The following analyses are planned:
Analysis 1. Validated food frequency and lifestyle questionnaires for each study participant. Dietary information must be recorded for the last five days prior to study recruitment. Dietary and lifestyle information will be recorded by the study dietitian in an epilepsy database developed by the Charité and will be included in our analysis.
Analysis 2. Clinical characterisation of epilepsy for the first two groups. Data collected during routine epilepsy care will be recorded in a standardised questionnaire: manifestation, seizures, seizure calendar, epilepsy / epilepsy syndrome, possible structural, genetic and autoimmune causes, developmental diagnostics. All patients are followed clinically by Prof. Kaindl’s medical team and all patient data are recorded in an epilepsy database developed by the Charité. Other important information about the patients’ treatment with anticonvulsants drugs is also recorded and taken into account in our analysis.
Analysis 3. Stool microbiome composition and metabolomics for all groups. Stool samples will be collected cross-sectionally from each group and longitudinally from group 1. Stool samples will be collected from individuals in group 1 before KD initiation, twice in the first week after KD initiation, then once two weeks after KD initiation, and then every month after KD initiation for up to six months. Faeces are freshly collected in the OMNIgene®-GUT kit, in which microbial DNA can be stored at room temperature for up to 3 months from the time of collection, prior to analysis of microbial composition using metagenomics to identify changes in microbiota composition. Fecal DNA will be prepared using the QIAamp PowerFecal Pro DNA Kit (QIAGEN) according to the manufacturer’s instructions. Sequencing will be performed on the Novaseq Xplus platform (Illumina) using the NovaSeq X Series 25B Reagent Kit (300 Cycle) (Illumina) at an average depth of 7.5 Gbases per sample. A second faecal aliquot sample will be collected in OMNImet®-GUT optimised to perform untargeted and targeted metabolomics. Amino acids, lipids, fatty acids, ketone bodies (beta-hydroxybutyrate, BHB and acetoacetate), indoles and bile acids will be quantified.
Analysis 4. Determination of serum metabolites and inflammatory parameters. Blood samples will only be collected from participants in the fist two groups for untargeted and targeted metabolomics, measurements of inflammatory mediators such as cytokines and chemokines will be performed on blood serum using the Meso Scale Discovery platform.
Aim 2. Isolation and characterisation of KD-associated human bacterial consortia. Hypothesis: The success of KD treatment in people with drug-resistant epilepsy is related to the composition and function of the gut microbiota. Aim: To isolate and characterise the metabolic functions of commensal bacteria thriving in paediatric patients with epilepsy treated with KD and showing (a) seizure improvement, (b) no seizure effect or (c) seizure worsening after KD introduction. Work plan: Ex vivo isolation and sequencing of KD-associated human bacteria. After one to 12 weeks on the new diet, some participants in group 1 will respond to the KD intervention and show an improvement in seizures (KD responders, >50% of the treated patients, with at least 50% of seizures improving -group 1A-), some will show neither improvement nor worsening of seizure evolution (KD non-responders, about 30% of the patients -group 1B-), while others will experience worsening of the disease after KD introduction(KD worse-responders, 5-10% of the patients -group 1C-). 3-5 subjects/group will be selected according to the development of seizures after KD consumption and a faecal sample will be collected within three months (for groups 1B and 1C) or six months (for group 1A) after the start of KD. This sample will be collected in the GutAlive (MicroViable Therapeutics) kit and sent immediately to the laboratory. This aliquot will be used for in vitro culture of the bacteria. in an anaerobic chamber. Each bacterial culture will be tested by Gram staining to assess the purity, and the genomic DNA is extracted to perform whole genome sequencing on the Pacific Biosciences (PacBio) Sequel machine at the Genomics Core Facility (MDC, Berlin). In this way, we will have the complete genome sequence of the identified bacteria, which will allow us to know all the genes expressed by each bacterium. This approach will allow us to select the bacteria that can be grown in vitro from the different groups of participant.
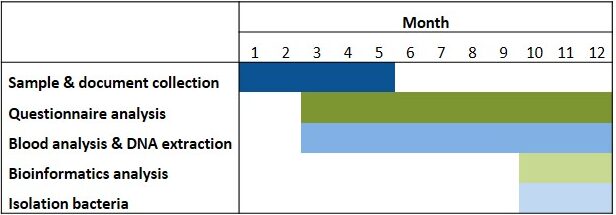
Table 1. Timeline. Isolation of bacteria will be possible depending on the development of the aim 1 of the research project.
Abbreviations. ASM: anti-seizure medicines; DRE: drug-resistant epilepsy; FatsQ: fats-type questionnaire; FFQ: food frequency questionnaire; KD: ketogenic diet; LifeSQ: lifestyle questionnaire; QOLIE 31: quality of life in epilepsy questionnaire
References
- Afrizal A, Hitch TCA, Viehof A, Treichel N, Riedel T, Abt B, […], Kohlheyer D, Overmann J, Clavel T. Anaerobic single-cell dispensing facilitates the cultivation of human gut bacteria. Environ Microbiol. 2022; 24(9):3861-3881.
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1990; 40(4):445-452.
- Cai QY, Zhou ZJ, Luo R, Gan J, Li SP, Mu DZ, Wan CM. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr. 2017; 13(6):528-536.
- Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021; 13(6):1-21.
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019; 16(8):461-478.
- De Caro C, Leo A, Nesci V, Ghelardini C, di Cesare Mannelli L, Striano P, […], Citraro R, De Sarro G, Russo E. Intestinal inflammation increases convulsant activity and reduces antiepileptic drug efficacy in a mouse model of epilepsy. Sci Rep. 2019; 9(1):1-10.
- Díaz-Marugán L, Rutsch A, Kaindl AM, Ronchi F. The impact of microbiota and ketogenic diet interventions in the management of drug-resistant epilepsy. Acta Physiol (Oxf). 2024; 240(3):e14104.
- Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy. Neurology. 2017; 88(3):296-303.
- Guerri G, Castori M, D’Agruma L, Petracca A, Kurti D, Bertelli M. Genetic analysis of genes associated with epilepsy. Acta Biomed. 2020; 91(13-S):e2020005.
- Guerrini R. Epilepsy in children. Lancet. 2006; 367(9509):499-524.
- Gómez-Eguílaz M, Ramón-Trapero JL, Pérez-Martínez L, Blanco JR. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: A pilot study. Benef Microbes. 2018; 9(6):875-881.
- He Z, Cui BT, Zhang T, Li P, Long CY, Ji GZ, Zhang FM. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s Disease: The first report. World J Gastroenterol. 2017; 23(19):3565-3568.
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: A 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001; 108(4):898-905.
- Iannone LF, Preda A, Blottière HM, Clarke G, Albani D, Belcastro V, […], Zara F, Russo E, Striano P. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother. 2019; 19(10):1037-1050.
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, […], Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009; 51(6):1069-1077.
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011; 365(10):919-926.
- Lyons L, Schoeler NE, Langan D, Cross JH. Use of ketogenic diet therapy in infants with epilepsy: A systematic review and meta-analysis. Epilepsia. 2020; 61(6):1261-1281.
- Matin N, Tabatabaie O, Falsaperla R, Lubrano R, Pavone P, Mahmood F, […], Di Mauro P, Cocuzza S, Vitaliti G. Epilepsy and innate immune system: a possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother. 2015; 11(8):2021-2029.
- Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2020; 6(6):CD001903.
- Mu C, Nikpoor N, Tompkins TA, Choudhary A, Wang M, Marks WN, Rho JM, Scantlebury MH, Shearer J. Targeted gut microbiota manipulation attenuates seizures in a model of infantile spasms syndrome. JCI Insight. 2022; 7(12):e158521.
- Newmaster K, Zhu Z, Bolt E, Chang RJ, Day C, Mhanna A, […], Mainali G, Carney PR, Naik S. A Review of the Multi-Systemic Complications of a Ketogenic Diet in Children and Infants with Epilepsy. Children. 2022; 9(9):1372.
- Oliver KL, Scheffer IE, Bennett MF, Grinton BE, Bahlo M, Berkovic SF. Genes4Epilepsy: an epilepsy gene resource. Epilepsia. 2023; 64(5):1368-1375.
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. 2018; 173(7):1728-1741.e13.
- Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: A population-based study in a Western European country. Epilepsia. 2008; 49(7):1230-1238.
- Rho JM and Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022; 18(6):333-347.
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020; 11:25.
- Singh G and Sander JW. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020; 105:106949.
- Sirven JI. Epilepsy: A Spectrum Disorder. Cold Spring Harb Perspect Med. 2015; 5(9):a022848.
- Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr. 2004; 9(2):98-101,106-109.
- Sultana B, Panzini MA, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, […], Jetté N, Josephson CB, Keezer MR. Incidence and Prevalence of Drug-Resistant Epilepsy: A Systematic Review and Meta-analysis. Neurology. 2021; 96(17):805-817.
- Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016; 131(2):211-234.
- Yan N, Xin-Hua W, Lin-Mei Z, Yi-Ming C, Wen-Hui L, Yuan-Feng Z, Shui-Zhen Z. Prospective study of the efficacy of a ketogenic diet in 20 patients with Dravet syndrome. Seizure. 2018; 60:144-148.