Identification of a gut microbiota signature in patients with drug-resistant epilepsy upon ketogenic diet treatment
Principle Investigator

Dr. Laura Díaz Marugán
Scientific interest within the context of the graduate college:
Our research project entitled “Identification of a gut microbiota signature in patients with drug-resistant epilepsy upon ketogenic diet treatment” aims to characterize the composition of the gut microbiota of patients affected by drug-resistant epilepsy undergoing ketogenic diet treatment, with the ultimate goal of developing probiotics as therapeutic tool. This research is characterized by combining both the clinical analysis of patients with epilepsy (encephalogram, NMRI, epigenetic changes and questionnaires on lifestyle and quality of life) and the molecular and cellular analysis of patients’ blood and stool. This project stands out for its strong clinical and experimental approach in close collaboration with the Clinic for Paediatrics and Neurology, Charité. The successful candidate will benefit from a training in which he/she will be able to put into practice his/her knowledge of the medical field and learn concepts, techniques and strategies of experimental research in the laboratory, paving the way to better understand how we could modulate the course of epilepsy via microbiota-diet modulation.
In this research project, the successful candidate will be able to:
- manage human sampling collection for research purposes.
- learn about the ketogenic diet as a therapy in the clinics
- learn about patient’s group stratification according to the different profiles in response to the treatment
- prepare and analyze questionnaires to track dietary habits, lifestyle habits, and quality of life
- extract and quantify genomic DNA from human stools
- get knowledge in microbial composition bioinformatic analysis
Project description:
Epidemiology and social impact of epilepsy and drug-resistant epilepsy (DRE): Epilepsy is one of the most common neurological disorders, affecting more than 50 million people worldwide and 6 million people in Europe (Singh & Sander, 2020; Sirven, 2015). It affects people of all ages and is characterized by epileptic seizures (Berg et al., 1999; Fiest et al., 2017; Guerrini, 2006; Sirven, 2015). Uncontrolled seizures can lead to developmental delay, cognitive deficits, memory and learning difficulties. They can also cause sudden unexpected death in people with epilepsy (SUDEP) (Sperling, 2004). Current treatments include anti-seizure medicines (ASM), diet, epilepsy surgery and stimulation devices. ASM can control seizures in about two-thirds of people with epilepsy. However, one-third of patients with epilepsy cannot be controlled by two or more correctly selected and dosed ASM, what is called drug-resistant epilepsy (DRE) (Kwan et al., 2009, 2011; Picot et al., 2008; Sultana et al., 2021).
The microbiota-gut-brain axis: a modulator for epileptogenesis: The causes of epilepsy include structural, genetic, infectious, metabolic and immune factors (Guerri et al., 2020; Matin et al., 2015; Vezzani et al., 2016; Oliver et al., 2023; Rho & Boison, 2022). The gut-brain axis (GBA) has also been implicated in this respect (Iannone et al., 2019). Gut microbiota produces a variety of compounds that can affect brain function, such as neurotransmitters and metabolites, like short-chain fatty acids (SCFAs) (Chen et al., 2021; Dalile et al., 2019; Silva et al., 2020), some of which have shown anticonvulsant effects in rodents (De Caro et al., 2019; Mu et al., 2022; Olson et al., 2018). In patients, studies have confirmed that bacterial-based approaches, such as probiotics or microbiota-modifying treatments, can reduce seizure frequency. Indeed, the pilot usage of a mixture of probiotics (eight bacterial subspecies of Lactobacillus, Bacteroides and Streptococcus) reduced seizure frequency and improved the quality of life in patients with DRE (Gómez-Eguílaz et al., 2018). Similarly, fecal microbiota transplantation (FMT) for the treatment of Crohn’s disease (CD) symptoms ameliorated seizures frequency in a 22-year-old woman with a 17-year history of epilepsy, who stopped taking ASM after the FMT (He et al., 2017). Despite these promising results, the mechanisms by which the microbiota may affect the development of seizures in patients are completely unclear.
Ketogenic diet in the management of DRE: Alternative treatments for DRE include dietary treatments such as the ketogenic diet (KD) (Hemingway et al., 2001; Lyons et al., 2020). KD is a diet based on a high fat intake accompanied by moderate protein, and very low or no carbohydrate content, usually in a 3:1 or 4:1 ratio of caloric intake from lipids to both protein and carbohydrate together, and with a normal total energy intake. Restricting carbohydrate intake forces the metabolism to replace the preferred energy source, glucose, with fatty acids, which are oxidized in the liver to form ketone bodies. Although the classical KD has been used in epilepsy for many years and its efficacy in reducing seizures has been confirmed in several studies, especially when introduced in young age (Martin-McGill et al., 2020), the mechanism behind its action is not well understood in humans. In particular, it is not known why this dietary intervention has an anti-seizure effect in a proportion of the patients, while in others there is poor or no effect (Martin-McGill et al., 2020) or even worsening of seizure frequency or appearance of adverse effects (Cai et al., 2017; Newmaster et al., 2022; Yan et al., 2018). Although the exact mechanism is not yet known, several research studies in both mice and human have shown a link between the gut microbiota and the effects of the KD, topic that we recently discussed (Díaz-Marugan et al., 2024).
Hypothesis: We hypothesize that the success of KD treatment in patients with drug-resistant epilepsy is related to the composition and function of the patient’s gut microbiota, so it would be possible to use the bacterial characteristics as biomarker to assess KD efficacy and as a probiotic tool to improve the response in those patients with a low KD-responsive profile. To test our hypothesis, our objectives are:
Aim 1: Identification of a KD-specific bacterial signature.
- To identify differences in bacterial composition and function in a large cohort of patients who respond or do not respond to KD.
- To track bacterial changes throughout the KD intervention and correlate them with seizure improvement or worsening.
- To analyze the duration of the effect of the ketogenic diet on both seizure improvement and gut microbiota changes even after the end of the intervention.
Aim 2: Isolation of bacteria associated with amelioration of seizure upon KD introduction.
- To isolate the most representative bacteria from KD responder patients.
Aim 3: Preclinical Model.
- To dissect the mechanisms of action of specific bacteria isolated from KD responders in gnotobiotic preclinical mouse models of epilepsy.
The successful candidate for this fellowship will focus on addressing aim 1. However, depending on the progress of the project (Table 1) it will be possible to start the experiments related to aim 2 (isolation of potential beneficial bacteria associated with a positive outcome of KD). This will be performed in the anaerobic chamber of our lab, through the cultivation of the bacteria from the human feces’ samples and in collaboration with Dr. Thomas Clavel (Uniklinik RWTH Aachen, Germany) using methods for single-cell dispensing of bacteria from human samples, as reported (Afrizal et al., 2022).
Table 1. Timeline. Isolation of bacteria will be possible depending on the development of the aim 1 of the research project.
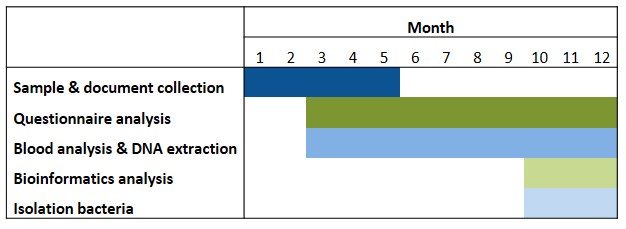
Abbreviations: ASM: anti-seizure medicines; DRE: drug-resistant epilepsy; FatsQ: fats-type questionnaire; FFQ: food frequency questionnaire; KD: ketogenic diet; LifeSQ: lifestyle questionnaire; QOLIE 31: quality of life in epilepsy questionnaire
References
- Afrizal A, Hitch TCA, Viehof A, Treichel N, Riedel T, Abt B, […], Kohlheyer D, Overmann J, Clavel T. Anaerobic single-cell dispensing facilitates the cultivation of human gut bacteria. Environ Microbiol. 2022; 24(9):3861-3881.
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1990; 40(4):445-452.
- Cai QY, Zhou ZJ, Luo R, Gan J, Li SP, Mu DZ, Wan CM. Safety and tolerability of the ketogenic diet used for the treatment of refractory childhood epilepsy: a systematic review of published prospective studies. World J Pediatr. 2017; 13(6):528-536.
- Chen Y, Xu J, Chen Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. 2021; 13(6):1-21.
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019; 16(8):461-478.
- De Caro C, Leo A, Nesci V, Ghelardini C, di Cesare Mannelli L, Striano P, […], Citraro R, De Sarro G, Russo E. Intestinal inflammation increases convulsant activity and reduces antiepileptic drug efficacy in a mouse model of epilepsy. Sci Rep. 2019; 9(1):1-10.
- Díaz-Marugán L, Rutsch A, Kaindl AM, Ronchi F. The impact of microbiota and ketogenic diet interventions in the management of drug-resistant epilepsy. Acta Physiol (Oxf). 2024; 240(3):e14104.
- Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy. Neurology. 2017; 88(3):296-303.
- Guerri G, Castori M, D’Agruma L, Petracca A, Kurti D, Bertelli M. Genetic analysis of genes associated with epilepsy. Acta Biomed. 2020; 91(13-S):e2020005.
- Guerrini R. Epilepsy in children. Lancet. 2006; 367(9509):499-524.
- Gómez-Eguílaz M, Ramón-Trapero JL, Pérez-Martínez L, Blanco JR. The beneficial effect of probiotics as a supplementary treatment in drug-resistant epilepsy: A pilot study. Benef Microbes. 2018; 9(6):875-881.
- He Z, Cui BT, Zhang T, Li P, Long CY, Ji GZ, Zhang FM. Fecal microbiota transplantation cured epilepsy in a case with Crohn’s Disease: The first report. World J Gastroenterol. 2017; 23(19):3565-3568.
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: A 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001; 108(4):898-905.
- Iannone LF, Preda A, Blottière HM, Clarke G, Albani D, Belcastro V, […], Zara F, Russo E, Striano P. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev Neurother. 2019; 19(10):1037-1050.
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, […], Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009; 51(6):1069-1077.
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011; 365(10):919-926.
- Lyons L, Schoeler NE, Langan D, Cross JH. Use of ketogenic diet therapy in infants with epilepsy: A systematic review and meta-analysis. Epilepsia. 2020; 61(6):1261-1281.
- Matin N, Tabatabaie O, Falsaperla R, Lubrano R, Pavone P, Mahmood F, […], Di Mauro P, Cocuzza S, Vitaliti G. Epilepsy and innate immune system: a possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother. 2015; 11(8):2021-2029.
- Martin-McGill KJ, Bresnahan R, Levy RG, Cooper PN. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev. 2020; 6(6):CD001903.
- Mu C, Nikpoor N, Tompkins TA, Choudhary A, Wang M, Marks WN, Rho JM, Scantlebury MH, Shearer J. Targeted gut microbiota manipulation attenuates seizures in a model of infantile spasms syndrome. JCI Insight. 2022; 7(12):e158521.
- Newmaster K, Zhu Z, Bolt E, Chang RJ, Day C, Mhanna A, […], Mainali G, Carney PR, Naik S. A Review of the Multi-Systemic Complications of a Ketogenic Diet in Children and Infants with Epilepsy. Children. 2022; 9(9):1372.
- Oliver KL, Scheffer IE, Bennett MF, Grinton BE, Bahlo M, Berkovic SF. Genes4Epilepsy: an epilepsy gene resource. Epilepsia. 2023; 64(5):1368-1375.
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell. 2018; 173(7):1728-1741.e13.
- Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: A population-based study in a Western European country. Epilepsia. 2008; 49(7):1230-1238.
- Rho JM and Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022; 18(6):333-347.
- Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020; 11:25.
- Singh G and Sander JW. The global burden of epilepsy report: Implications for low- and middle-income countries. Epilepsy Behav. 2020; 105:106949.
- Sirven JI. Epilepsy: A Spectrum Disorder. Cold Spring Harb Perspect Med. 2015; 5(9):a022848.
- Sperling MR. The consequences of uncontrolled epilepsy. CNS Spectr. 2004; 9(2):98-101,106-109.
- Sultana B, Panzini MA, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, […], Jetté N, Josephson CB, Keezer MR. Incidence and Prevalence of Drug-Resistant Epilepsy: A Systematic Review and Meta-analysis. Neurology. 2021; 96(17):805-817.
- Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016; 131(2):211-234.
- Yan N, Xin-Hua W, Lin-Mei Z, Yi-Ming C, Wen-Hui L, Yuan-Feng Z, Shui-Zhen Z. Prospective study of the efficacy of a ketogenic diet in 20 patients with Dravet syndrome. Seizure. 2018; 60:144-148.