Characterization of long-term immobility induced thromboprotective mechanisms in immune cells
Principle Investigator

Dr. Marco Witkowski
Scientific interest within the context of the graduate college:
Disease prevention is taking a central role in tackling the burden of cardiovascular disease in our aging society. Thrombotic cardiovascular diseases such as venous thromboembolism, stroke and myocardial infarction are main drivers of morbidity and mortality worldwide. A central pathomechanism in these thrombotic cardiovascular diseases is termed thromboinflammation and arises from a deleterious dysregulation of an evolutionary conserved host defense mechanism involving platelets, the innate immune and coagulation system. There is an unmet clinical need for a better mechanistic understanding of thromboinflammation, in order to novel regulatory mechanisms that may pave the way for potential therapeutics, biomarkers and preventive measures. By pursuing this aim we established an across-species approach to investigate the paradox that long-term immobility during hibernation in brown bears and patients with spinal cord injury (SCI) does not increase the risk of thrombosis.
Our research acts at the interface between immunology, cardiovascular science and preventive medicine by taking advantage of an interdisciplinary translational research approach.
Project description:
Introduction: In this project we will characterize long-term immobility-induced thromboprotective mechanism arising within the innate immune cell compartment (i.e. neutrophils and monocytes) as key player in thromboinflammation and thrombotic cardiovascular diseases.
In the initial phase of venous thrombus development, primed monocytes expose TF to induce the extrinsic coagulation pathway while activated neutrophils release NETs as prothrombotic scaffolds triggering activation of the intrinsic pathway that both culminate in excessive fibrin formation. This is paralleled by release of neutrophil elastase that degrades TF pathway inhibitors thereby suspending antithrombotic mechanisms and aggravating thrombus formation. However, during later stages of thrombosis, neutrophils regulate thrombus resolution by modulating plasmin-mediated fibrin degradation and metalloproteinase-mediated reorganization of the thrombus matrix. In preliminary data we found that indicate that central neutrophil functions, such as bacterial phagocytosis, are attenuated during hibernation. Discovery proteomics identified 132 differentially regulated neutrophil proteins under hibernation suggesting a causal link between altered protein expression and cell function (Figure 1A). We hypothesize that hibernation (i.e. long-term immobility) induced cellular traits in neutrophils and monocytes that regulate their thrombotic und thromboinflammatory capacity.
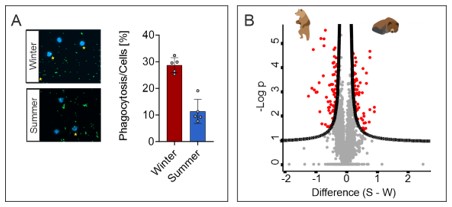
Figure 1. (A) Bacterial (green) phagocytosis by neutrophils (blue, DAPI). (B) Discovery proteomics identified 132 differentially regulated proteins (red dots) in neutrophils between hibernation and active period.
Aim 1: Defining and characterizing the role of longterm-immobility induced immune cell traits in vitro and in vivo in the context of thromboinflammation. Therefore, we will use our across-species approach (i.e. hibernating bears, SCI patients and bed-resting individuals) to study immune cell function by multicolor-flow cytometry, co-culture assays, state-of-the-art microscopy as well as in vivo model of thrombosis.
Aim 2: Investigating the role of long-term immobility-induced immune cell traits in patients with venous thromboembolic disease and myocardial infarction.
References
- Thienel M, Muller-Reif JB, Zhang Z, Ehreiser V, Huth J, Shchurovska K, […], Mann M, Massberg S, Petzold T. Immobility-associated thromboprotection is conserved across mammalian species from bear to human. Science. 2023; 380(6641):178-187.
- Petzold T, Zhang Z, Ballesteros I, Saleh I, Polzin A, Thienel M, […], Schulz C, Hidalgo A, Massberg S. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity. 2022; 55(12):2285-2299.e7.
- Petzold T, Thienel M, Dannenberg L, Mourikis P, Helten C, Ayhan A, […], Schulz C, Kelm M, Polzin A. Rivaroxaban Reduces Arterial Thrombosis by Inhibition of FXa-Driven Platelet Activation via Protease Activated Receptor-1. Circ Res. 2020; 126(4):486-500.